The NUS Guangzhou Research Translation and Innovation Institute is committed to building a cross-disciplinary and cross-field research exchange platform to promote the effective transformation and application of research achievements. We will regularly share cutting-edge research results and dynamics with you in this column, and welcome exchanges and discussions!
The research introduced in this article comes from the Biocatalysis and Green Chemistry Laboratory at the National University of Singapore. It was completed by PhD student Willy Wei Li See under the guidance of laboratory director Associate Professor Zhi Li during his doctoral studies, and the article was published in ACS Catalysis (Willy W.L.See and Zhi Li, ACS Catal. 2023, 13, 13215-13224). This research will be continued by PhD student Li Li, who is a scholarship recipient from the NUS Guangzhou Research Translation and Innovation Institute.
Research Background
2-Biarylpropanoic acids form the structure of many widely used non-steroidal anti-inflammatory drugs (NSAID), such as ketoprofen, flurbiprofen, and felbinac. The enantioselective synthesis of these drugs is highly desired: the S-enantiomers possess anti-inflammatory properties, and some R-enantiomers are strong candidates for Alzheimer’s disease treatment. Conventional chemical synthesis of these acid-containing molecules involves highly toxic cyanide-based chemistry or carbonylation, followed by a kinetic resolution with a limitation of 50% maximum yield. Their direct chemical asymmetric synthesis from alkenes is also hampered by poor enantioselectivity or regioselectivity. Therefore, we developed the green and efficient synthesis of NSAID drugs and other 2-Biarylpropanoic acids via biocatalysis.
Research Content
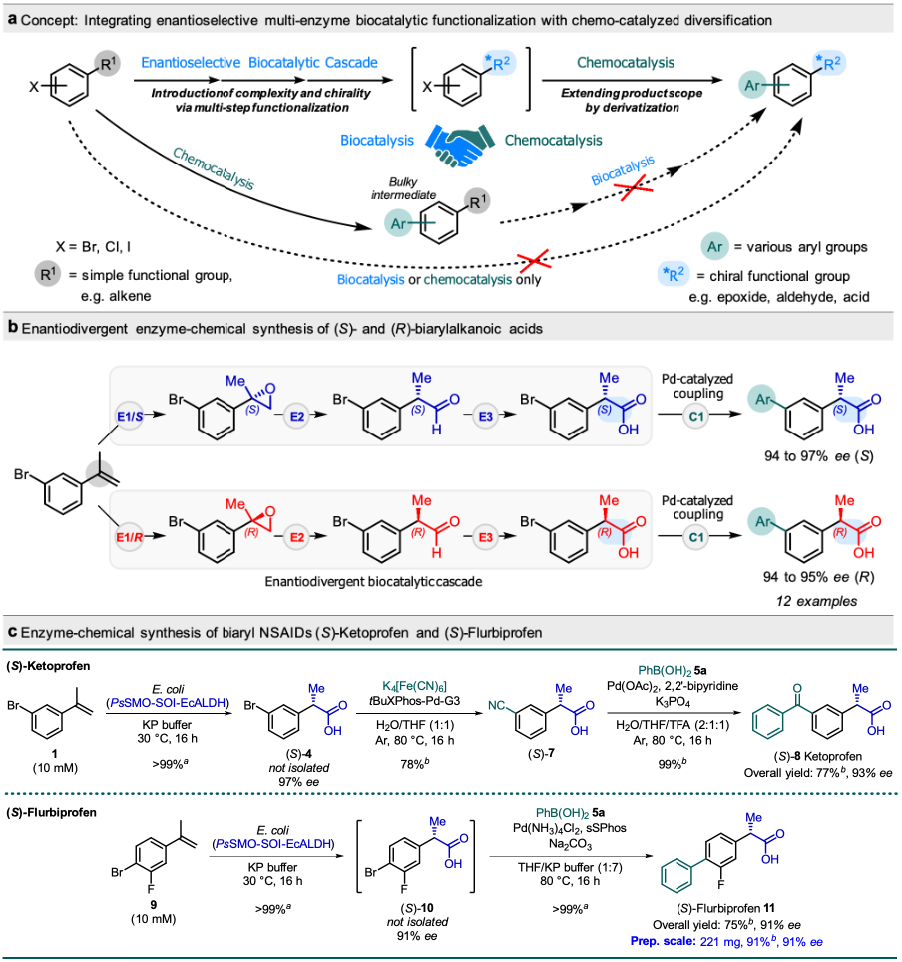
Figure 1 a-c
We achieved the green and efficient synthesis of NSAID drugs and other 2-biarylpropanoic acids by the development of a novel enzyme-chemo catalysis concept (Figure 1a) for complex asymmetric functionalization of simple substrates. The concept consists of integrating multistep biocascades to introduce functionality and enantioselectivity, with robust chemocatalysis to diversify the product scope. It was utilized for the enantiodivergent synthesis of a diverse panel of (S)- and (R)-2-biarylpropanoic acids from aryl alkenes by combining enantiodivergent biocatalytic cascades with Pd-catalyzed cross-coupling reactions under mild aqueous conditions (Figure 1b). The reaction was also extended to produce NSAID drugs (S)-ketoprofen and (S)-flurbiprofen (Figure 1c), and various useful chiral and nonchiral biarylacetic acids (Figure 2).
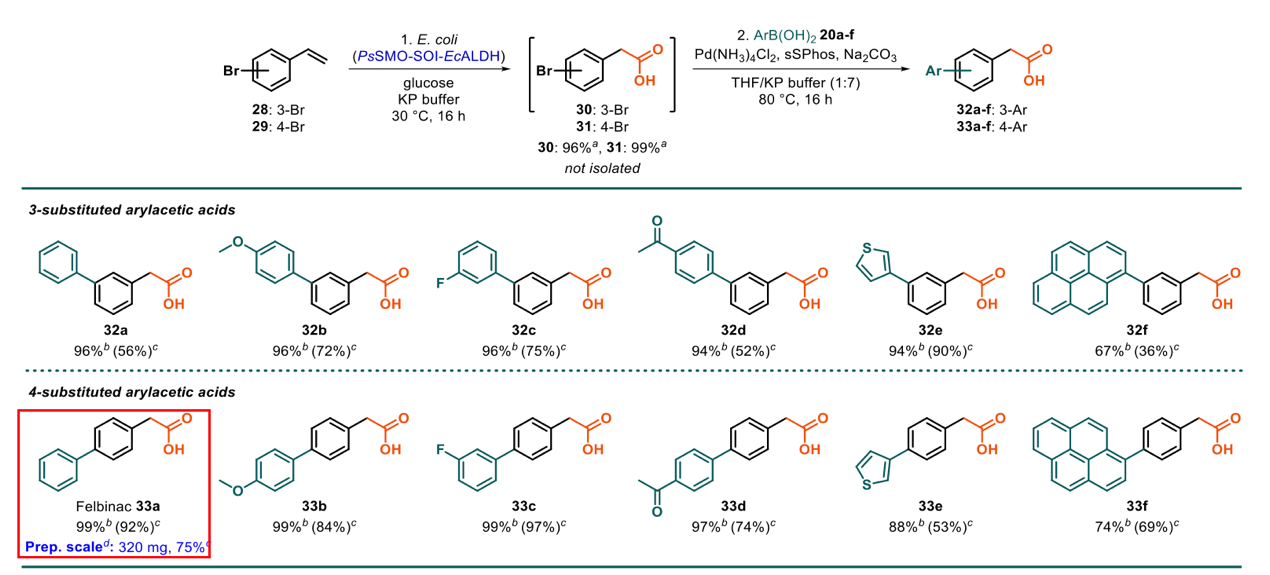
Figure 2
The integration of multistep biocascades with chemocatalysis via an enzyme-chemo sequence enables new synthetic routes to produce structurally diverse chiral products. Such a synthesis is unachievable by either type of catalyst alone: biocascades have a limited substrate scope to produce diverse products, while there is a lack of chemical counterpart for complex asymmetric functionalization. Such a synthesis is also unachievable by chemo-enzymatic reaction via a chemo-enzyme sequence: while chemical derivatization of the substrate is easy, the later biocascade would be restricted by substrate specificity.
Key Achievements or Significant Discoveries
A “multienzyme cascade-chemocatalysis” concept was developed for asymmetric transformation to produce complex chiral chemicals with structural diversity from simple substrates. It integrates biocatalytic cascades to introduce complexity and chiral functionality with chemical diversification to extend the product scope, enabling useful and challenging synthesis which cannot be achieved via biocatalysis or chemo-catalysis alone, or a chemo-enzyme sequence.
The concept was successfully demonstrated through the enantiodivergent synthesis of both (S)- and (R)-enantiomers of a series of 2-biarylpropanoic acids, an important class of compounds including many widely used NSAID drugs, from easily available bromoaryl alkenes by engineering enantio-complementary epoxidation−isomerization−oxidation biocatalytic cascades and combining with Pd-catalyzed cross-coupling. This is the first report combining multistep (>2) enantio-selective biocatalytic cascades with chemo catalysis for complex asymmetric synthesis. The enzyme-chemical reaction successfully produced 12 structurally diverse examples of (S)- and (R)-2-biarylpropanoic acids, in 94−97% ee and up to >99% conversion, as well as NSAID drug (S)-flurbiprofen in 91% ee and 91% conversion. Furthermore, combining the biocatalytic cascade with Pd-catalyzed cyanation and aryl addition converted 3-bromophenyl alkene to the NSAID drug (S)-ketoprofen in 93% ee and 77% yield. Overall, this method provides direct, green, and efficient synthesis of chiral 2- biarylalkanoic acids from simple substrates.
The enzyme-chemical strategy was also used to functionalize easily-available 3- and 4-bromostyrenes into 12 structurally varied biarylacetic acids, including NSAID drug felbinac, achieving >90% yields in most cases. The method is practical and also greener than the conventional toxic cyanide-based acid synthesis.
Our established concept harnesses the best of both worlds of biocatalysis and chemocatalysis, combining enantioselective biocatalytic cascades and robust chemocatalysis. It is potentially useful for the general asymmetric synthesis of different types of chiral molecules with diverse structures from simple substrates and could encourage new development in this direction to create other innovative and practical synthetic routes.
Introduction of Project Leader and Project Team

Prof. Zhi Li
This research was published in ACS Catalysis (Willy W.L. See and Zhi Li, ACS Catal. 2023, 13, 13215-13224), carried out by Willy W.L. See in his Ph.D thesis research under the supervision of Prof. Zhi Li. The work will be continued by Li Li, a new Ph.D student on the NUS GRTII Scholarship program.
The research of Prof. Li’s group focuses on advanced biocatalysis for green and sustainable manufacturing of chemicals and pharmaceuticals, production of bio-based fuels and chemicals, and development of functional and smart materials. (Group website: https://cheed.nus.edu.sg/stf/chelz/people.html)
The NUS Guangzhou Research Translation and Innovation Institute focuses on seven major areas: smart cities, information and communication, electronics technology, advanced manufacturing, artificial intelligence, biosciences, and fintech. Relying on NUS's world-class research capabilities, the institute has introduced dozens of professors, associate professors, and assistant professors from NUS as academic leaders, leading local research teams to actively promote research transformation, and postdoctoral training programs.
We look forward to working with global experts, entrepreneurs, and policymakers to stimulate innovative thinking, explore the limitless possibilities of scientific research, and establish a cross-disciplinary and cross-field ecosystem for scientific research and innovative applications.


