The NUS Guangzhou Research Translation and Innovation Institute is committed to building a platform for interdisciplinary and cross-domain scientific research exchange, promoting the effective transformation and application of research achievements. We will regularly share cutting-edge research results and updates in this section. We welcome your engagement and discussions!
This article introduces the research on unveiling and targeting cell wall modeling for the development of novel antibiotics. This research was conducted by the group led by Assistant Professor Luo Min from the Department of Biological Sciences at the National University of Singapore. Established in mid-2021, this is a vibrant and innovative research team. The group adopts a multidisciplinary approach, combining techniques from biochemistry, cell biology, computational biology, and structural biology, such as cryo-electron microscopy (cryo-EM), to explore fundamental questions about bacterial cell division and intercellular communication. Its main objective is to develop natural peptide antibiotics to address the critical challenge of multidrug resistance in infectious diseases and to design antiviral peptide drugs to combat future epidemics. Assistant Professor Luo Min is a PhD supervisor at the NUS Guangzhou Research Translation and Innovation Institute.
Research Background
Cell wall remodeling and cell-cell communication are essential processes in microbiology, playing pivotal roles in bacterial cell division and serving as prime targets for antibiotic development. By exploring the underlying mechanisms of these processes, our research seeks to identify novel targets for antibiotic intervention, addressing the urgent global challenge of antimicrobial resistance (AMR). A key focus is the development of innovative antibiotics such as lanthipeptides, naturally occurring antimicrobial peptides that bacteria produce to inhibit the growth of competitors. Unlike traditional antibiotics, which often lead to AMR, lanthipeptides target bacterial membranes, offering a promising alternative with a lower risk of resistance development.
Research content and achievements
Our major contributions in this field are highlighted in five high-impact publications from the past year:
1. Li J.W., He Y.T., Xu X., Alcorlo M., Shi J., Roper D.I., Hermoso J., Sham L.T., Luo M. eLife. 2024. https://elifesciences.org/reviewed-preprints/94336
Here, we provide a comprehensive structural analysis for the first time of the FtsEX system in E. coli, showing how ATP binding activates the complex to regulate AmiB, an enzyme critical for cell wall cleavage.
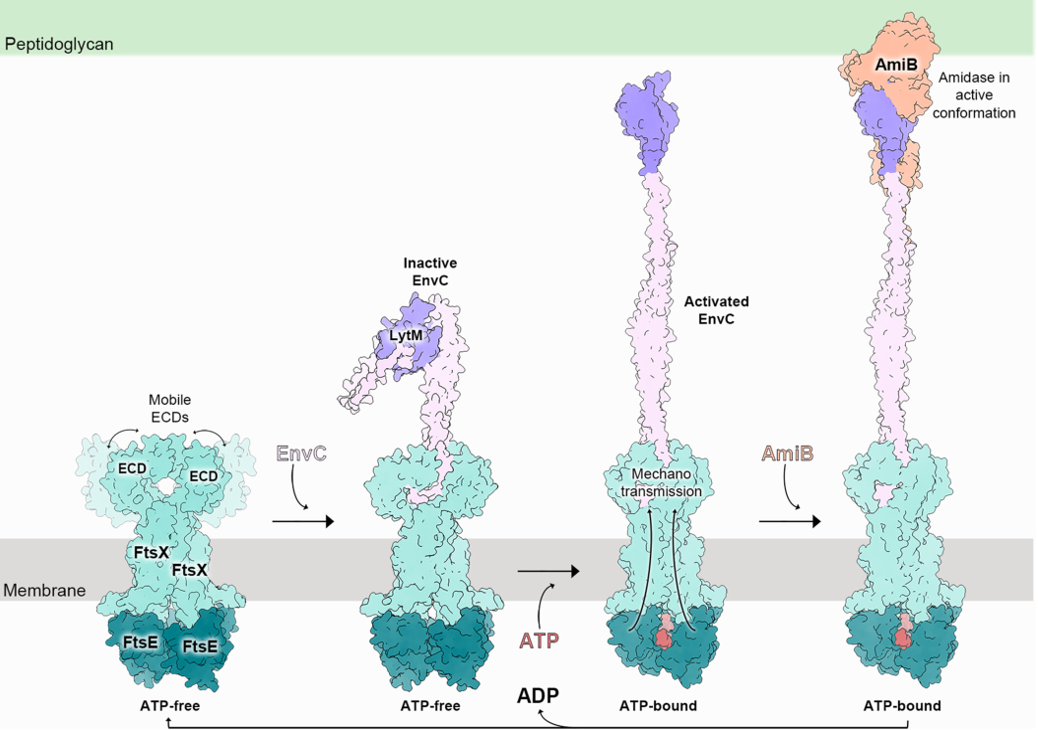
2. Xu X., Li J.W., Chua W.Z., Pages M.A., Shi J., Hermoso J.A., Bernhardt T., Sham L.T., Luo M. Proceedings of the National Academy of Sciences USA. 2023. DOI: 10.1073/pnas.2301897120
This publication reveals how the FtsEX-EnvC complex activates amidase enzymes to regulate peptidoglycan hydrolysis during bacterial division, uncovering a conserved mechanism across bacterial species that holds potential for antibiotic development.
3. Li J.W., Xu X., Shi J., Hermoso J., Sham L.T., and Luo M. Nature Communications. 2023. DOI: 10.1038/s41467-023-43770-6.
This study explores the regulation of RipC, a key enzyme involved in bacterial cell division, by the FtsEX system in Mycobacterium tuberculosis, providing valuable insights into potential antibiotic targets within the bacterial division machinery.
4. Yu L., Xu X., Chua W.Z., Feng H., Ser Z., Shao K., Shi J., Wang Y., Li Z., Sobota R.M., Sham L.T., and Luo M. Nature Communications. 2023. DOI: 10.1038/s41467-023-42852-9
This groundbreaking study reveals the structural basis for peptide secretion in quorum sensing within Gram-positive bacteria. We identified a critical binding site on the ComA enzyme, providing a promising target for the development of antibacterial therapies aimed at disrupting bacterial communication.
5. Li Y.F., Shao K., Li Z.X., Shi J., Xiao Y.B., Luo M. Nature Communications. 2024. DOI: 10.1038/s41467-024-51600-6
In this publication, we introduce a new subclass of lanthionine synthetase KC enzymes, unveiling their structure and function. We detail the formation of functional dimers in different peptide-binding states, providing critical insights into lanthipeptide modification. This study highlights the therapeutic potential of these enzymes in developing novel antibiotics.
In the future, the research group led by Assistant Professor Luo Min will continue to delve into the fundamental questions of bacterial cell division and intercellular communication. Driven by innovation, they aim to provide new solutions for global anti-infective therapy.
The NUS Guangzhou Research Translation and Innovation Institute primarily focuses on seven key areas: smart city, information and communication, electronic science and technology, advanced manufacturing, artificial intelligence, biological sciences, and financial technology. Relying on NUS’ world-class research capabilities, the Institute has engaged dozens of professors from NUS as academic leaders. These leaders guide local research teams in promoting scientific research, research translation, and postdoctoral training programs.
We look forward to working with global research experts, entrepreneurs, and policymakers to stimulate innovative thinking, explore the infinite possibilities of scientific research, and establish an interdisciplinary and cross-field ecosystem for scientific research and innovative applications.


